Namaste! Hope you are doing well and never have a need to take the “medicine” which I am today going to talk about.
This post is about Mukta Vati, an Ayurvedic medicine made by Patanjali with tall claims and the reality behind it. If you are the tik-tok or Insta generation of reader wanting a quick dump, go to the bottom of the post for a summary.
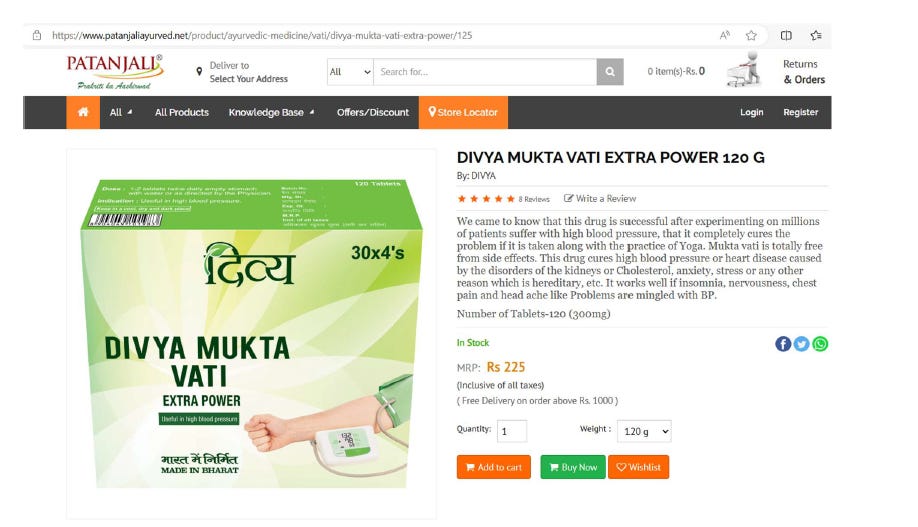
So, Patanjali claims: “We came to know that this drug is successful after experimenting on millions of patients suffering with high blood pressure. It completely cures the problem if taken along with the practice of Yoga. Mukta Vati is totally free from side effects”.
Wow, that is some claim. They have “experimented” on millions!? Drug innovators conduct trials, but Patanjali has experimented on millions. Then the question arises were these millions informed that they are being experimented on? People who participate in trials have to sign a consent form where they are informed about the trials, possible side effects and are monitored for the effect of the drug. Let’s go further.
I then looked into the CTRI database where every manufacturer has to register for trials before a medicine can be released into the market. Surprise, surprise!
The trial was only registered in 2020 as seen above. Now lets look at the recruitment for the trial.
Huh!!? So basically, only 50 participants were recruited, 50. Fifty.
Was the trial completed? No.
Where is the trial data? Nowhere published. If you, dear reader have seen any published data for this trial, please inform in the comments section below.
I still did not give up. I filed an RTI with Ayush ministry for more information. The reply I got is typical governmental Could-not-care-less-go-to-hell.
“In this regard, it is to inform that the sought information relates to the State licensing authority, Uttarakhand. Hence, it is suggested that you may directly contact the concerned Licensing Authorities (Ayush) for desirable information in accordance with the Para 3(iv) of guidelines prescribed by Ministry of Personnel”
Basically they passed the buck to Uttarakhand authorities, despite the fact that this drug is sold all over the country which I reminded them in my appeal. Uttarakhand Ayush is untraceable on any website to file an RTI.
However, the Supreme court cracked down on Patanjali for their false advertising! Wonderful act. However, the Hindu/Right wing took offense and pointed out to Rooh Afza as a prime example why “their” products are not touched. Typically silly. Who buys Patanjali products? I suspect the non-Rooh Afza customers? So if a harmful product, or atleast a product of dubious effect is stopped who benefits?
After the SC ruling, Patanjali removed all their unfounded advertisements.
Summary:
Patanjali claimed a million people were experimented upon for the Mukta Vati medicine for lowering blood pressure.
In reality, 50 patients were recruited for a trial registered in 2020. The outcome of the trial is unknown.
RTI in India is really a joke. Passing the buck is the norm.